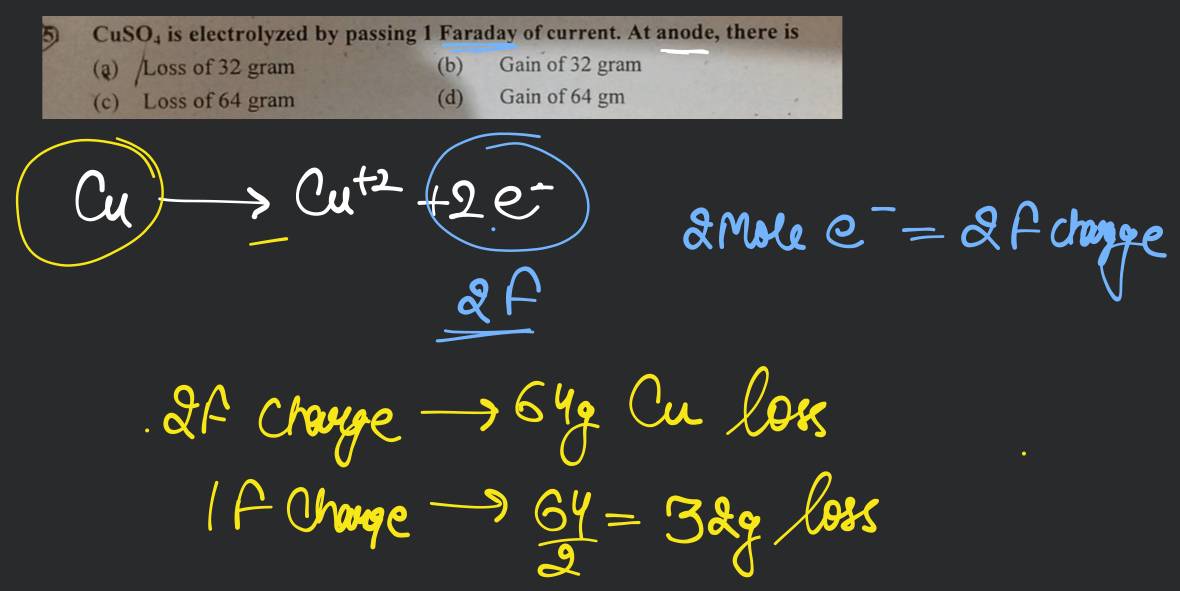Electrolysis of water with 1 faraday electricity gives | CLASS 12 | ELECTROCHEMISTRY | CHEMISTRY... - YouTube
1.Explain and give e.g. on 'how 1 Faraday will always deposits 1 gm equivalent mass of a substance.'

1 mol of charge = 1 Faraday = 96500 coulombs. Represent the cell and the standard emf of the cell having the following reaction. 2Cr(s) +3Ca(aq) = 2Cr + (aq) + 3Cd()
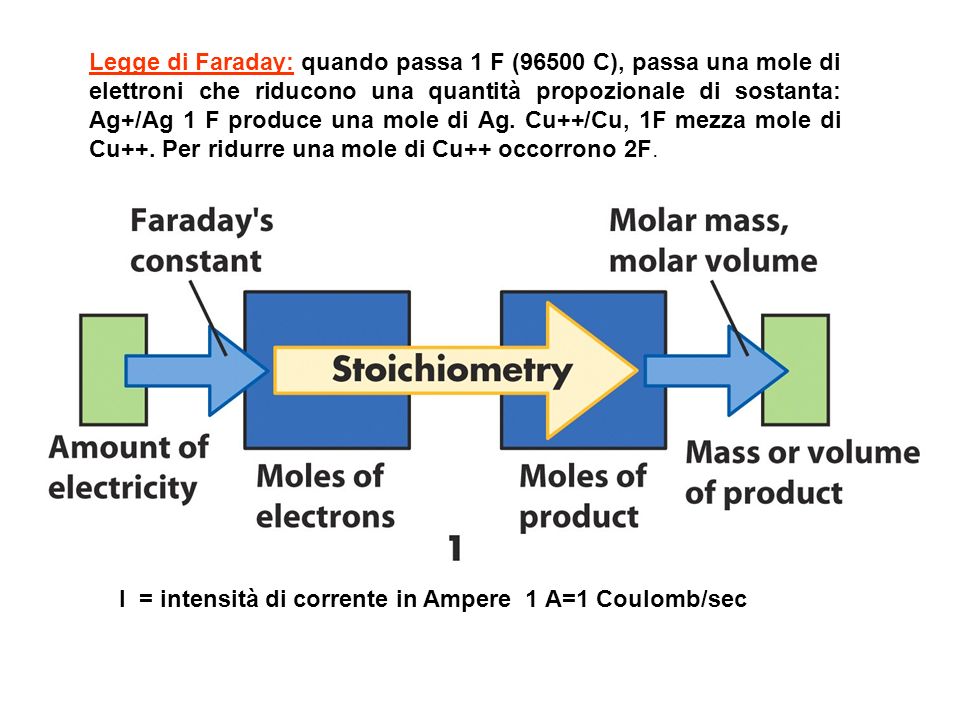
Legge di Faraday: quando passa 1 F (96500 C), passa una mole di elettroni che riducono una quantità propozionale di sostanta: Ag+/Ag 1 F produce una mole. - ppt scaricare

1 faraday charge is passed through aq solutions of AgNO3, Cuso, and Fecig. The ratio of g equivalents of Ag(s) : Cu(s): Fe(s) deposited is (1)/1: 1:1 (2) 6:3:2 (3) 1:2:3 (4) 1:2:1
FARADAY'S LAW. NaCl (s) → Na + (l) + Cl – (l) E° R = V E° O = V 2 Cl - (l) → Cl 2(g) + 2e - 2 Na + (l) + 2e - → 2 Na (s) Electrolytic cell. - ppt download
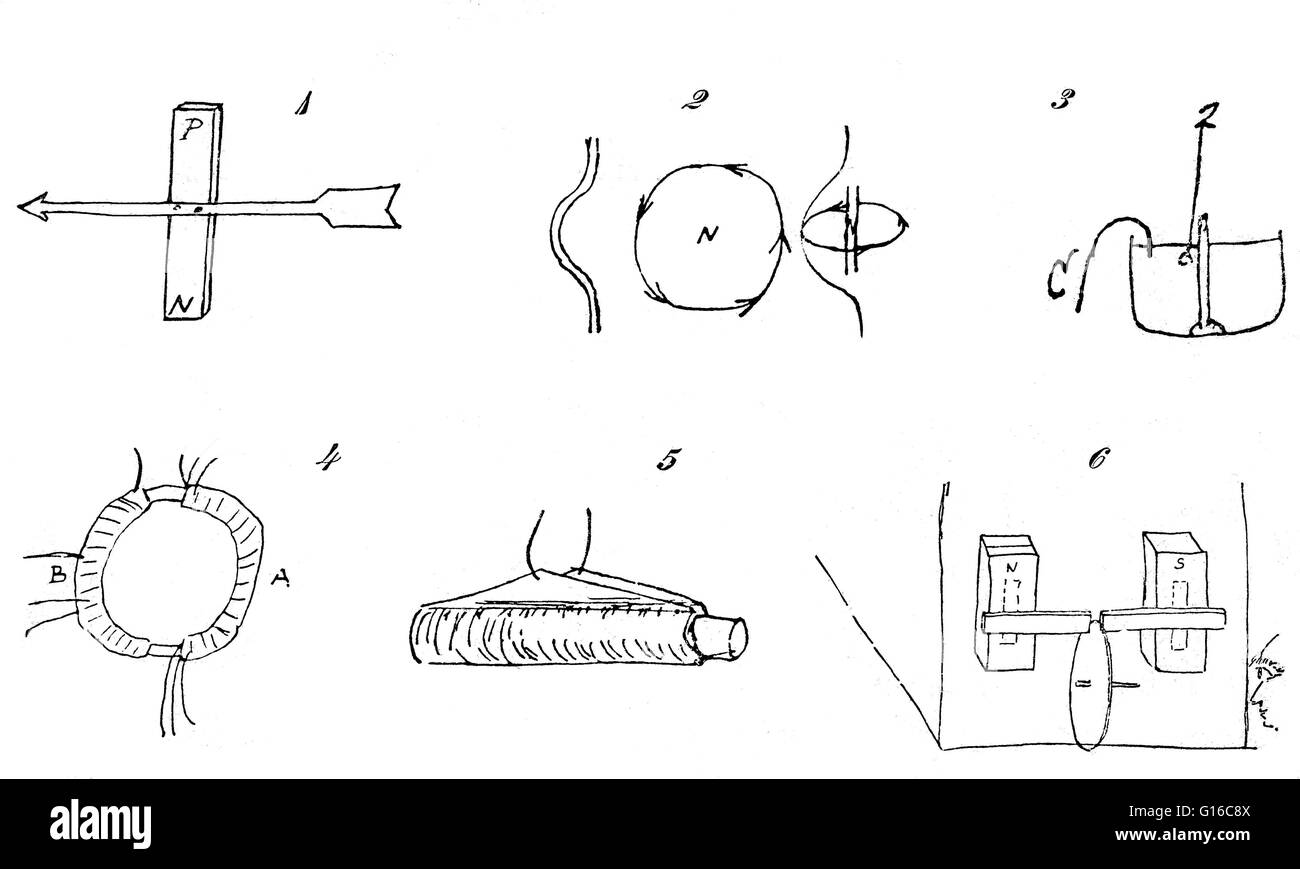
Schizzi da Faraday il diario della mostra la progressione dei suoi esperimenti elettromagnetici. 1) Egli ripete Oersted in seguito alla scoperta del 1820 che un ago magnetico eccepire nei pressi di un

Cyber Nichel Rame 1 Faraday Tessuto EMF Schermatura 50 "x 3' Materiale di blocco del segnale - Plain Weave : Amazon.it: Casa e cucina

The mass of the substance deposited by 1 faraday of electricity is equal to 11 grams. The value of electrochemical equivalent is: A. 11 B. 11 x 96500 - Correct Answers 11 96500 D. data insufficient
the amount of copper deposited by 1 faraday current will be maximum in an acidic solution of 1l of 1.1MCu2Cl2 2.2MCu(NO3)2 3.5M CuSO4 4.5M Cu3(PO4)3 5.10M CuF2